
#MYSTERY OF #ORANGE #AURORAS: A recent display of auroras over #Canada has experts scratching their heads. The mystery? They were orange:
"This was a first for me," says Harlan Thomas, who photographed the display over Sibbald Pond west of Calgary, Alberta, on Oct. 19th. "The orange was sublime, just incredible. The pillars in the center stayed there glowing for more than 20 minutes."
Auroras aren't supposed to be orange. Consider the following: Auroras get their colors from atoms and molecules in Earth's atmosphere. During geomagnetic storms, energetic particles rain down from space, striking the air and causing it to glow. Red, green, purple and even pink are common signs of excited oxygen and nitrogen.
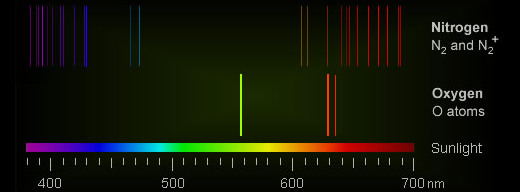
The problem is, there's nothing in the air capable of making bright orange. Theoretically, nitrogen and oxygen (N2, N2+, and O2+) can produce emissions at orange wavelengths. However, these emissions are very weak compared to other colors produced by the same molecules. Any orange should be overwhelmed.
#Aurora colors produced by atoms and molecules in Earth's atmosphere.
The answer may be hiding in plain sight. Take another look at Thomas's photo. Bright red auroras appear on top, overlapping #green auroras lower down. #Red and green mixing together may have produced the #yellow-orange #glow.
Indeed, aurora physicist Kjellmar Oksavik of the University of Bergen in Norway believes that's the correct explanation:
"Red auroras are formed by low-energy electrons colliding with atomic oxygen at high altitudes (200-400 km). Here, oxygen atoms are excited into a quantum state called O(1D), where they can emit a red photon at 630.0 nanometers," Oksavik says.
"Green auroras are formed by higher-energy electrons penetrating deeper and colliding with atomic oxygen at lower altitudes (100-150 km)," he continues. "Here, oxygen atoms are excited into a state called O(1S), where they emit a green photon at 557.7 nanometers."
"In between, there can be a mixing of the two processes, which fools the camera and eye to believe that it is orange. In reality, it is both red and green at the same time," he says.
Oksavik points out one more thing in Harlan Thomas's photo: "It beautifully reveals the alignment of Earth’s magnetic field. The bright pillar in the center is a textbook example of a very tall auroral ray. These are aligned along the magnetic field and caused by a broad energy spectrum of electrons [raining down from space]. Slower electrons collide high up (red light), while more energetic electrons travel further down into a much denser atmosphere (green light)." The overlap naturally produces a yellow-orange glow--no mystery molecule required.
https://spaceweather.com/


















