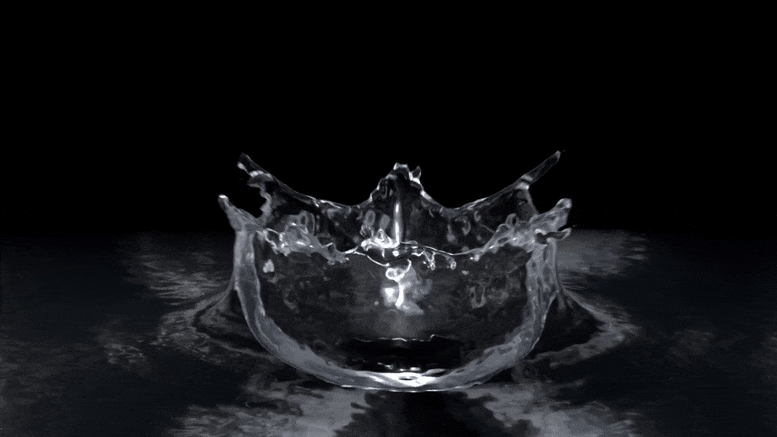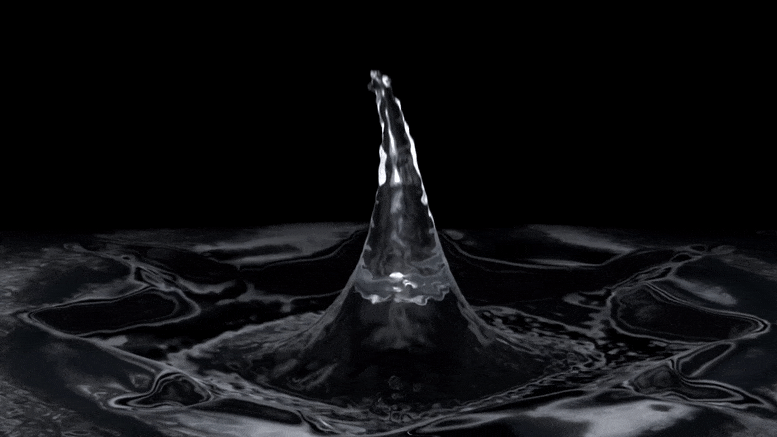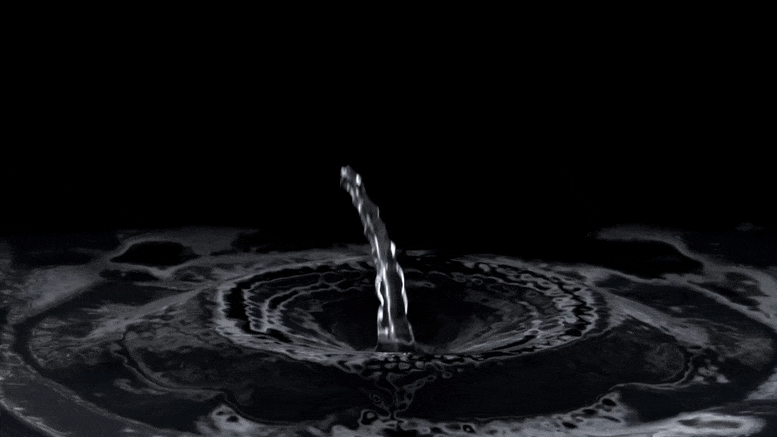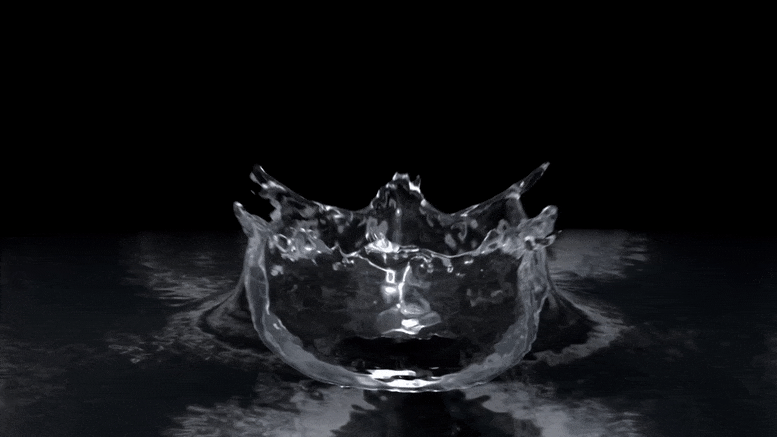
"Rust-stained irrigation pipes hint at lack of nitrate in groundwater." "Oh, we don't have to worry about that area. They have red pivots." "On its face, the anecdote was clear enough. The 'red' in question, Troy Gilmore knew, was rust. The pivots, meanwhile, were center pivots: elevated irrigation piping that rotates around a central point to distribute water in circular patterns most evident from 30,000 feet, where irrigated crops resemble massive green checkers crowding the pastoral checkerboard of the Corn Belt."
"As for the worry? That would be nitrate, a fertilizer-derived compound that can leach into groundwater and, if consumed above certain concentrations via drinking water, pose threats to human health."
"Still, the associate professor at the University of Nebraska-Lincoln found the offhand comment a bit surprising. Even with his extensive background in hydrology, Gilmore had never heard of any connection between rusty pivots and groundwater nitrate. But Marty Stange, the environmental supervisor with Hastings Utilities, was in the middle of explaining a reverse-osmosis water treatment facility that had recently gone into operation."
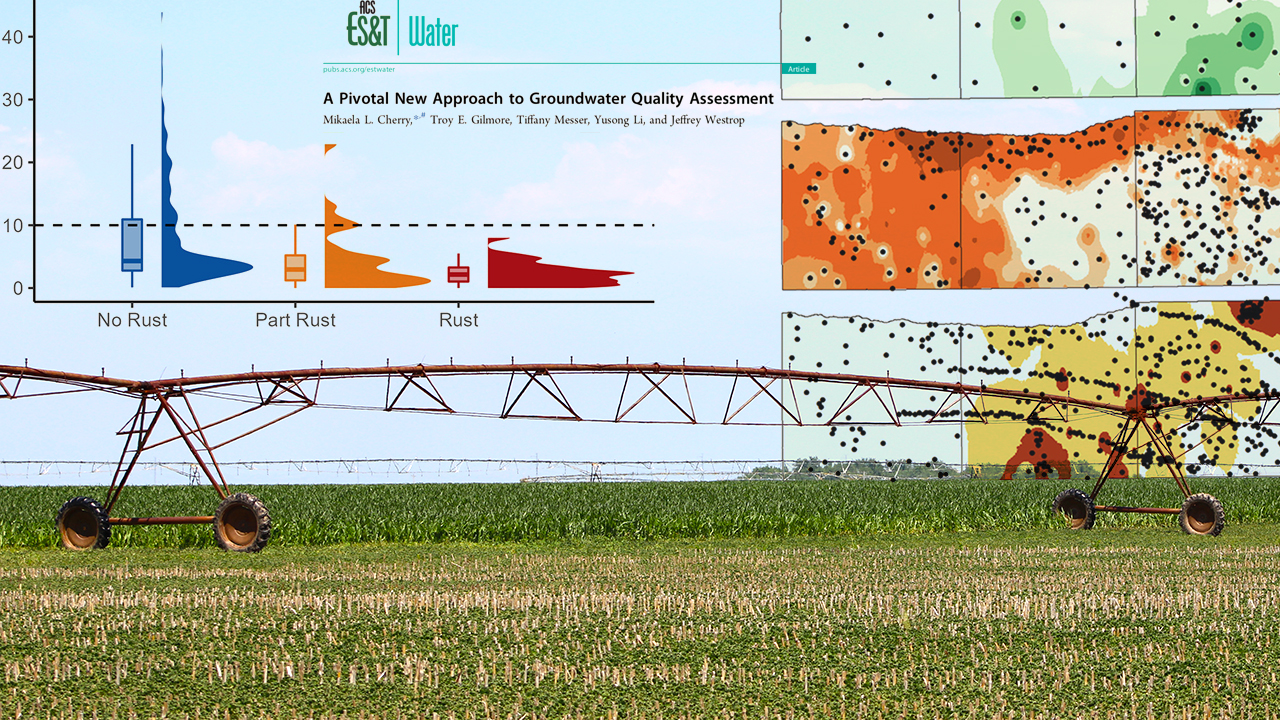
"Over a three-day span, Mikaela Cherry and her sister drove across three countries of rural Nebraska while snapping photos of the region's widespread irrigation pipes. Now, after dozens of hours peering at satellite imagery, compiling spreadsheets of data and driving the country roads of south-central Nebraska, Cherry and her colleagues have good reason to suspect that Stange was right. According to their research, red center pivots -- specifically, those totally stained with reddish-brown iron pumped up from the aquifers below -- seem to signify an absence of nitrate in whatever groundwater flows through their pipes."
The reverse isn't true, though: a non-rusty pivot doesn't mean the groundwater is high in nitrate. It doesn't tell you anything.
She went on to pour through Google Earth and classify 700 of the pivots into one of three categories: full-rust, part-rust or no-rust.
"After cross-referencing the locations of the pivots with their groundwater nitrate concentrations, Cherry discovered that none of the 76 groundwater wells feeding into the full-rust pivots contained nitrate above the 10 mg/L threshold. In fact, the average nitrate concentration of those sources was just 2.4 mg/L. Most, though not all, of the groundwater supplied to the part-rust pivots also sat below the nitrate threshold, with an average concentration of 4.5 mg/L but a maximum of nearly 23 mg/L."
If you're wondering what the connection is between rust and groundwater nitrate, well, you're out of luck, as nobody knows. The researchers have a guess, though, involving microorganisms, such as bacteria, that live in soil and groundwater. If microorganisms prefer to discharge unwanted electrons to nitrate instead of iron, then they go to iron only if there's no nitrate, and that's the form of iron that oxidizes and turns to rust if it is exposed to oxygen, which happens as soon as the water is pumped to the surface. But if there's no iron in the groundwater, then the rust doesn't happen regardless of nitrates. But this is just a guess and future research will tell if it's correct.
Rust-stained irrigation pipes hint at lack of nitrate in groundwater | Nebraska Today
#discoveries #chemistry #agriculture